TMB Harmonization Project
Tumor Mutational Burden Harmonization Project
The Friends of Cancer Research (Friends) Tumor Mutational Burden (TMB) Harmonization Project has contributed to significant advances in cancer research. By unifying the way TMB is measured across various laboratories, this initiative identified approaches to enhance consistency in the evaluation of the genetic mutations of tumors. This standardization plays a crucial role in optimizing the use of TMB for identifying patients best suited to benefit from immunotherapy, enhancing personalized care, and improving patient outcomes.
Learn more about our current portfolio of Research Partnerships.
Historically, cancer treatment involved chemotherapy, which attacks dividing cells throughout the body, including many cells that are not cancerous. In recent years, scientific innovation has facilitated the development of targeted therapies, which can precisely attack cancer cells that have specific molecular features known as biomarkers, potentially providing more effective and safer treatments for patients. It is necessary to identify which patients have the biomarker and may benefit from specific targeted therapies. To help identify which patients have a specific biomarker, diagnostic developers have created tests, also known as assays.
One biomarker of interest is Tumor Mutation Burden (TMB). Research suggests that patients with a higher number of tumor mutations are more likely to benefit from targeted treatment with immune checkpoint inhibitors (ICIs). Specifically blocking checkpoint proteins allows for immune cells (T cells) to attack cancerous cells. Prior to initiation of the TMB Harmonization project, there was a lack of standardization for TMB calculation and reporting. Findings from the project highlight different tests reported different measurements, and since there is no singular way of calculating TMB it may be difficult to use as a biomarker. To achieve consistency and accurate reporting across tests, it was imperative to standardize assays to arrive at clinically meaningful results and support informed decision-making for patients.
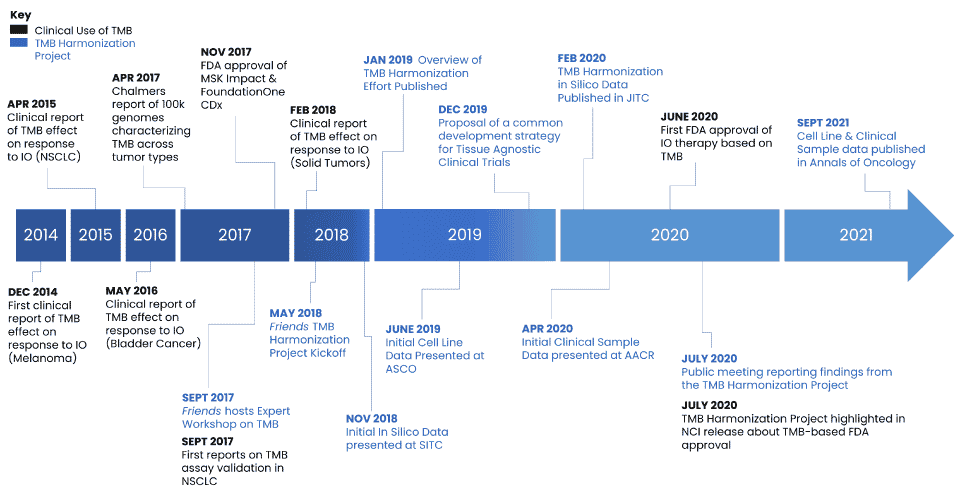
Friends initiated a unique collaboration with key stakeholders including pharmaceutical companies, assay developers, FDA, and academics in September 2017 to discuss the variability in how TMB is defined, analyzed, and used in clinical practice, and the need for establishing industry standards. Throughout the course of the TMB Harmonization Project, Friends hosted discussions with project participants to develop consensus on a methodological approach to compare TMB assays, to develop a calibration tool to promote reproducibility and comparability across assays, and to provide recommendations for a clinical cutoff to support evaluation of TMB for clinical trial enrollment using a common strategy. Findings from each phase of the project were disseminated through public workshops, conferences, and manuscripts.
The results of the TMB Harmonization Project informed consensus recommendations on a standardized approach to measure TMB. If assay developers use these strategies, providers and patients will have more consistent results regardless of which assay they use to measure TMB. This project will help ensure consistent identification of patients who are likely to respond to immunotherapies and, ultimately, improve patient outcomes.
Project Outcomes
- In 2021, Phase 2 findings were published in Annals of Oncology.
- In 2020, Friends presented data at AACR that provided findings from the analysis of clinical samples.
- In 2020, results from Phase 1 of the project were published in the Journal for ImmunoTherapy of Cancer (JITC).
- In 2019, Friends presented initial data from the cell line analysis at the ASCO Annual Meeting.
- Building on initial findings from Phase 1, in 2019, Friends and the Quality Assurance Initiative Pathology (QuIP) published an Overview of the TMB Harmonization Project which proposes recommendations to support consistent TMB estimation and reporting methods in clinical samples across assays and centers.
- In 2018, Friends presented the initial results from the Phase 1 In Silico Analysis at the Society for ImmunoTherapy of Cancer (SITC) Annual Meeting.
Project Partners
ACT Genomics, AstraZeneca, Biodesix, Brigham & Women’s Hospital, Bristol Myers Squibb, Caris Life Sciences, College of American Pathologists, Columbia University, the European Organisation for Research and Treatment of Cancer (EORTC), EMD Serono, Inc., the U.S. Food and Drug Administration (FDA), Foundation Medicine, Inc., Genentech, Inc., Genomic Testing Cooperative, Guardant Health, Inc., Hartwig Medical Foundation, Illumina, Inc., Intermountain Precision Genomics, Johns Hopkins University, Massachusetts General Hospital, Merck & Co., Inc., Memorial Sloan Kettering Cancer Center, MD Anderson Cancer Center, National Cancer Institute (NCI), NeoGenomics Laboratories, Inc., OmniSeq, Personal Genome Diagnostics (PGDx), Pfizer, Inc., precisionFDA, Q2 Solutions, QIAGEN, Inc., Quality in Pathology (QuIP), Quest Diagnostics, Regeneron Pharmaceuticals, Roche Diagnostics, SeraCare, Thermo Fisher Scientific, Thrive, University of Heidelberg
