Real-World Evidence Portfolio
Real World Data (RWD) is patient data routinely collected in electronic health records (EHRs), claims data, and registries that can provide valuable information about the real-world performance of cancer therapies and diagnostics. Unlike in traditional clinical trial settings where data are collected at pre-established timepoints and reported uniformly for trial participants, there is typically a lot of variation in the way RWD are reported within and across sources. Inconsistent definitions and data missingness in RWD present challenges to using these data sources to investigate treatment effectiveness, such as how the therapy impacts survival or can be used in certain patient populations. Strategies and methodologies for mitigating these factors and aligning RWD are needed to fully realize the potential of RWD.
Since 2017, Friends has facilitated collaboration among RWD vendors, pharmaceutical companies, government officials, and academics to advance the understanding and applications of RWD and real-world evidence (RWE) in oncology. These multistakeholder partnerships support alignment on best practices, provide a venue to develop and test methodologies for aligning and analyzing RWD, and help identify opportunities to proactively strategize on future use of RWD/E in oncology:
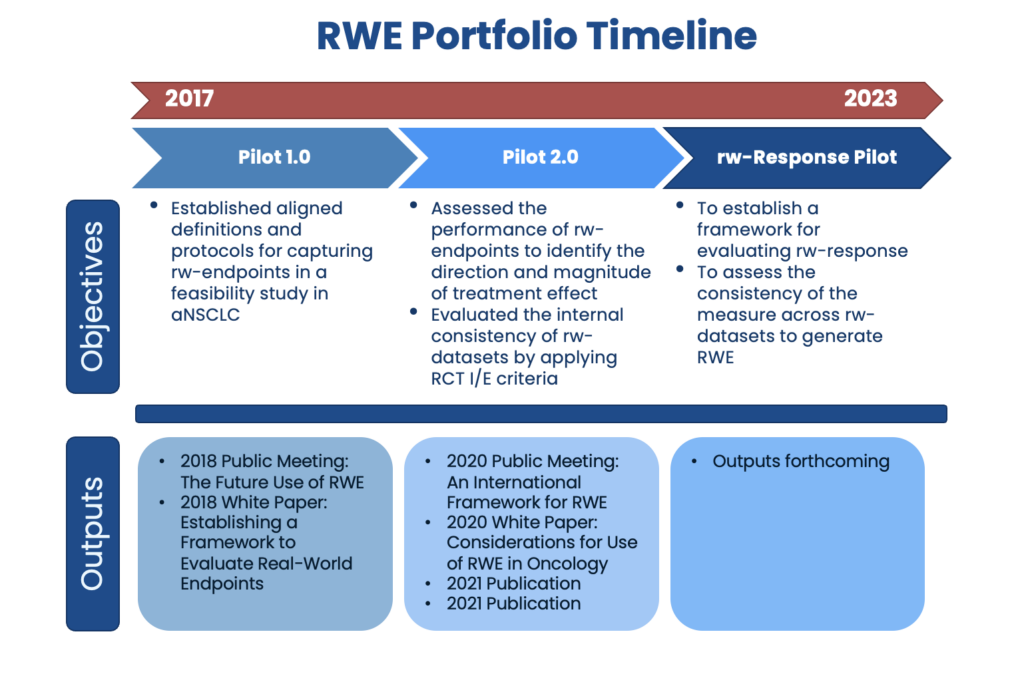
RWE Pilot 1.0 created a framework for aligning data sources and showed that implementing a harmonized protocol and set of definitions for necessary data elements supports identification of similar patient populations across data sources and extraction of several real-world endpoints.
RWE Pilot 2.0 applied the framework developed in Pilot 1.0 and showed that RWD can be used to generate similar results to clinical trials when measuring the treatment effect of chemotherapy compared with immunotherapy in patients with advanced non-small cell lung cancer (aNSCLC). The group developed a list of considerations for the design, conduct, and interpretation of RWD studies from different data sources.
rw-Response is an ongoing effort that uses lessons learned during Pilots 1.0 and 2.0. The rw-Response Pilot will establish a framework for measuring rw-response and assess the consistency of the measure across RWD sources in first-line regimens for patients with aNSCLC.
rw-Care is an ongoing effort to develop a framework for the use of RWD at point of care to improve patient outcomes from immunotherapy by mitigating treatment-related adverse events. The ongoing prospective pilot will assess whether improved immune-related adverse event management, facilitated by real-time access to patient-specific data, can result in improved patient care, reduced or fewer adverse events and/or healthcare utilization.
Traditional clinical trials, such as randomized controlled trials (RCTs), can take a long time and typically enroll a narrow patient population that represents a fraction of the patients who will likely receive the product in the real-world. RWD can generate supplemental evidence that reflects a larger and more diverse patient population than is included in clinical trials and address timely clinical questions including use of different treatments in standard of care practice and long-term safety. Aligning best practices and frameworks for aggregating and analyzing RWD will help to ensure RWE is high quality and reliable for supporting oncology drug development and regulatory decision-making and real-world use of products.
Portfolio Timeline
Project Outcomes
- Friends hosted a public meeting titled The Future Use of Real-World Evidence on July 10, 2018. The meeting white paper, Establishing a Framework to Evaluate Real-World Endpoints, details Pilot 1.0 and a framework for operationalizing and validating real-world evidence in advanced NSCLC.
- The 2020 white paper, Considerations for Use of Real-World Evidence in Oncology: Lessons Learned from Friends of Cancer Research Collaborations, identified the implications of dataset variability on measuring real-world endpoints and recommendations for developing a framework to guide future RWE studies through a harmonized protocol.
- Friends hosted a virtual event September 21-22, 2020, An International Framework for Real-World Evidence, sharing the RWE Pilot 2.0 results and discussing potential policy and regulatory needs presented by this work.
- Results of the RWE Pilot 2.0 were published in Clinical Pharmacology and Therapeutics:
- In June 2023, Friends presented a poster at the ASCO Annual Meeting.
- On September 11 2023, Friends hosed a public meeting titled Supporting the Use of RWD in Oncology Drug Development. The meeting presented results from the Friends real-world Response (rw-Response) Pilot. Click here to view the meeting presentation. A discussion document was also released, titled Considerations for Leveraging Real-World Endpoints in Oncology Drug Development.
Project Partners
Pilot 1.0: COTA, the U.S. Food and Drug Administration (FDA), Flatiron Health, IQVIA, Kaiser Permanente/Cancer Research Network (CRN), Mayo Clinic & OptumLabs, and National Patient Centered Cancer Research Network (PCORnet)/University of Iowa
Pilot 2.0: Aetion, American Society of Clinical Oncology (ASCO) CancerLinQ & ConcertAI, COTA, FDA, Flatiron Health, IQVIA, Kaiser Permanente, Mayo Clinic & OptumLabs, McKesson, National Cancer Institute (NCI) SEER-Medicare Linked Database, Syapse, Tempus AI Inc.
rw-Response Pilot: Ambra Health, ASCO, Columbia University, ConcertAI, COTA, FDA, Flatiron Health, Guardian Research Network, IQVIA, Ontada, Syapse, Tempus AI Inc.
