Molecular diagnostics are central to the delivery of personalized cancer care. Without these important tools, physicians would not be able to select the appropriate patients for targeted therapy, negating the advantages conferred by recent advances in genomics.
In the past fifteen years, there has been an explosion of new technologies to interrogate the genes and proteins that play a role in cancer growth and development. A quick Google search will verify this—dozens of labs and diagnostic companies are using a range of techniques to test for predictive markers in cancer.
This progress is promising but also a little dizzying. It is not fully clear how various testing platforms measure up to one another, although regular proficiency testing helps to confirm test accuracy. It is also difficult to say for sure how results from the most sophisticated tests should be interpreted, given that they may output tens, if not hundreds, of potentially actionable genomic markers.
Recognizing the importance of accurate, reliable, and efficient molecular testing, Friends of Cancer Research (Friends) collaborated on a research partnership with the Deerfield Institute in 2015 to better understand trends in the molecular diagnosis of cancer. The idea was to identify whether physician practices across the country were testing patients according to guidelines put out by leading clinical organizations, and whether barriers exist that prevent timely patient access to targeted therapies.
In 2015, the Deerfield Institute and Friends developed a physician questionnaire related to molecular testing in lung cancer. In developing this physician questionnaire, we sought to answer three questions about the use of molecular diagnostics:
- Is availability of adequate tissue samples a rate-limiting step in tumor molecular analysis?
- What is the uptake of next-generation sequencing platforms across practice settings and regions?
- How often is molecular testing performed too late to enable patients to be treated with a targeted therapy in the first-line setting?
Broadly, these questions address whether practices are adapting to a changing environment to allow molecular diagnostics to have their greatest impact on patient management. Below is a summary of the primary findings from the research. To read the full white paper, click here.
Survey Question:
When testing for genetic alterations in NSCLC, how many separate tissue biopsies are typically performed per patient over the course of his/her disease progression?
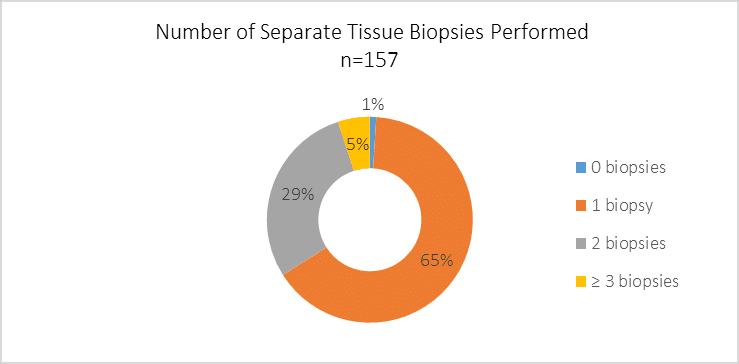
Many patients with lung cancer have small tissue specimens acquired through biopsies. Since some tissue is required initially to determine histology, there is sometimes limited tissue left over for use in molecular testing. There is often the possibility of performing additional biopsies, but these are invasive procedures and can be burdensome on patients. Thus, many observers have called for biopsy techniques that gather enough tissue for multiple purposes.
Survey Question:
How has the utilization of the following test formats changed in the past year, if at all?
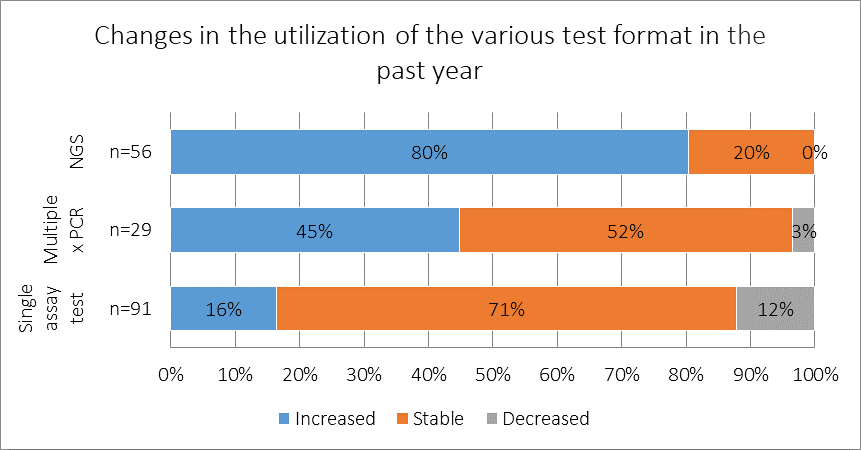
Among respondents who reported using NGS-based panels to test patients for lung cancer mutations, 80% reported that the rate of test utilization increased in their practice during the past year. Among the 91 respondents who reported using single assay tests, 71% reported that usage of this testing technique was stable in the past year, suggesting that, although use of newer formats is increasing, most practices are still heavily relying on single assay tests.
Survey Question:
Thinking of your EGFR and ALK positive patients, what proportion had their mutation discovered prior to 1st line chemotherapy, and what proportion during 1st line chemotherapy?
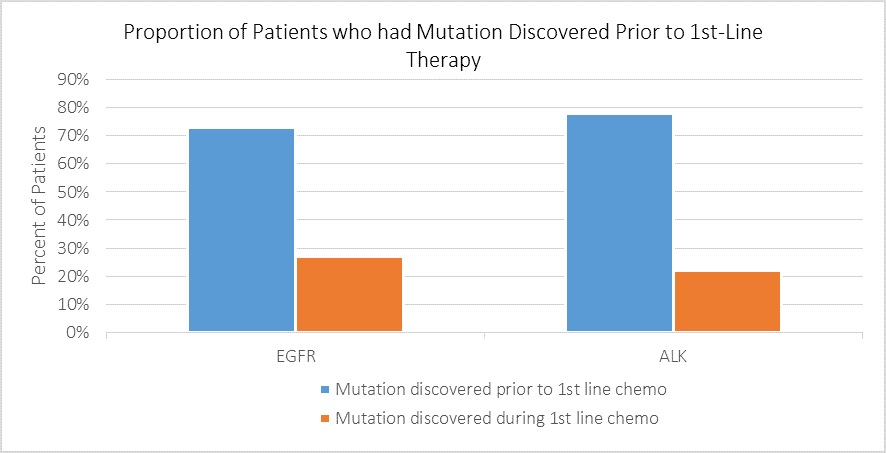
Respondents reported that among patients who tested positive for EGFR mutations and ALK rearrangements, 73% and 78%, respectively, had their mutation discovered prior to undergoing chemotherapy. This is a promising finding, meaning that the majority of patients do not begin less effective treatment before their eligibility for targeted therapy is known.
Please click here to read the full white paper. In addition, in 2016, Friends and the Deerfield Institute jointly published a study in the journal Personalized Medicine in Oncology drawing from data also collected through the research partnership described above. The 2016 study addressed a major policy issue—the regulation of laboratory developed tests (LDTs)—which was relevant to the FDA’s proposal to enhance oversight of these tests. Following publication of the study, Friends and the Deerfield Institute participated in a briefing on Capitol Hill to discuss policy implications of the work and educate the public about the regulation of molecular diagnostics.
Financial support for this research was provided by the Deerfield Institute, the internal research group at Deerfield Management Company, a healthcare investment firm dedicated to advancing healthcare through investment, information and philanthropy.
