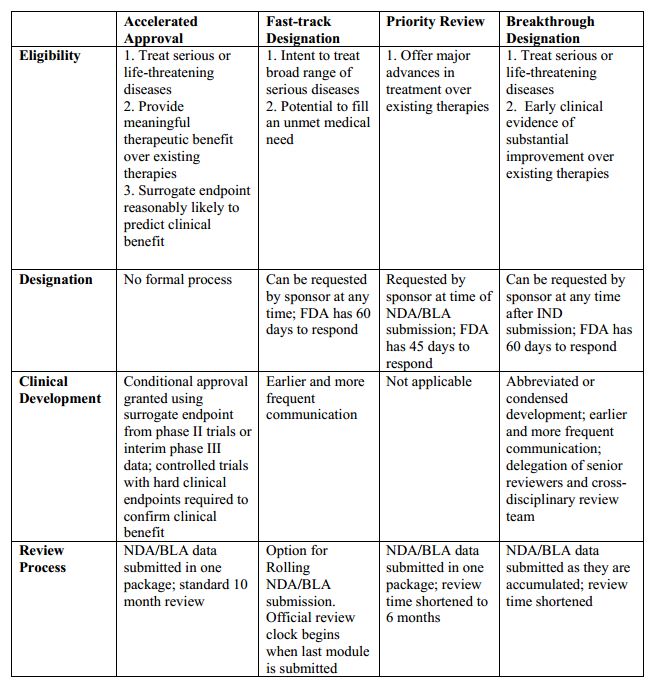
The U.S. Food and Drug Administration (FDA) attempts to review all drugs efficiently, but gives special consideration to therapies that treat serious or life-threatening diseases or have the potential to provide unusually large benefits to patients. The agency uses four distinct mechanisms to speed the development and availability of drugs treating serious or life-threatening conditions: Priority Review, Accelerated Approval, Fast Track, and Breakthrough Therapy. While they all aim to improve FDA efficiency, these approaches employ different selection criteria and target different parts of the drug development and approval process.
Priority Review:
- Requested alongside Biologics License Application (BLA) or New Drug Application (NDA) submission
- Cuts a drug’s FDA review period from ten months to six
- Drugs qualifying for Fast Track, Breakthrough Therapy, and Accelerated Approval can also be eligible for Priority Review
- More information about Priority Review
Fast Track:
- Requested as early as Investigational New Drug (IND) application and prior to BLA or NDA submission
- Intended for drugs that address unmet medical need by either treating a condition for which no other treatment exists or offering some substantial benefit over existing treatment
- Sponsors get extra opportunities to meet with FDA, discuss approval requirements and study design, and identify their most efficient path through drug development and review
- Sponsors may also gain access to rolling review, wherein portions of their marketing application may be reviewed before the complete application has been submitted
- More information about Fast Track
Breakthrough Therapy:
- Requested as early as IND application and preferably prior to end-of-Phase II meeting
- Similar to Fast Track, but Breakthrough drugs must show early clinical evidence of substantial improvement over existing therapies
- Same benefits as Fast Track, with an even greater emphasis on early meetings and coordination with experienced and senior FDA personnel
- Due to their large early clinical effect, Breakthrough drugs can sometimes skip portions of the standard FDA review process without compromising safety and efficacy standards
- More information on Breakthrough Therapy, Friends and Breakthrough Therapy
Accelerated Approval:
- Sponsors should discuss Accelerated Approval with FDA during development
- Intended for drugs with long-term endpoints, such as increased survival or decreased morbidity, that are difficult to measure efficiently in trials
- Allows approval based on surrogate endpoints—more easily measured outcomes that are reasonably likely to predict clinical benefit (e.g. tumor shrinkage can be used as a surrogate endpoint for survival benefit in some instances of cancer)
- Sponsors are required to confirm a drug’s efficacy in post-market clinical trials
- Priority Review, Fast Track, and Breakthrough drugs can also be eligible for Accelerated Approval
- More information on Accelerated Approval
For a more comprehensive overview of these programs, read the FDA’s own description here.
Here’s a quick comparison of these programs: